Abstract
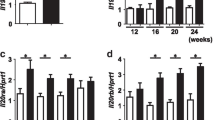
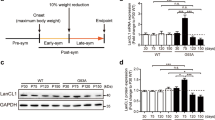
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by the loss of motor neurons in the brain and spinal cord. ALS neuropathology is associated with increased oxidative stress, excitotoxicity, and inflammation. We and others reported that the anti-aging and cognition-enhancing protein Klotho is a neuroprotective, antioxidative, anti-inflammatory, and promyelinating protein. In mice, its absence leads to an extremely shortened life span and to multiple phenotypes resembling human aging, including motor and hippocampal neurodegeneration and cognitive impairment. In contrast, its overexpression extends life span, enhances cognition, and confers resistance against oxidative stress; it also reduces premature mortality and cognitive and behavioral abnormalities in an animal model for Alzheimer’s disease (AD). These pleiotropic beneficial properties of Klotho suggest that Klotho could be a potent therapeutic target for preventing neurodegeneration in ALS. Klotho overexpression in the SOD1 mouse model of ALS resulted in delayed onset and progression of the disease and extended survival that was more prominent in females than in males. Klotho reduced the expression of neuroinflammatory markers and prevented neuronal loss with the more profound effect in the spinal cord than in the motor cortex. The effect of Klotho was accompanied by reduced expression of proinflammatory cytokines and enhanced the expression of antioxidative and promyelinating factors in the motor cortex and spinal cord of Klotho × SOD1 compared to SOD1 mice. Our study provides evidence that increased levels of Klotho alleviate ALS-associated pathology in the SOD1 mouse model and may serve as a basis for developing Klotho-based therapeutic strategies for ALS.








Similar content being viewed by others
Abbreviations
- ADAM:
-
A disintegrin and metalloprotease domain
- ALS:
-
Amyotrophic lateral sclerosis
- ALS TDI:
-
ALS Therapy Development Institute
- AD:
-
Alzheimer’s disease
- Axin 2:
-
Axin-related protein 2
- AHCs:
-
Anterior horn cells
- C9ORF72:
-
Chromosome 9 open reading frame 72
- CSF:
-
Cerebrospinal fluid
- CCR2:
-
C-C chemokine receptor type 2
- Cox-2:
-
Cyclooxygenase-2
- FALS:
-
Familial amyotrophic lateral sclerosis
- FGFR:
-
Fibroblast growth factor receptors
- FOXO:
-
Forkhead box O
- Fzd5:
-
Frizzled gene family encoding 7-transmembrane domain proteins
- GFAP:
-
Glial fibrillary acidic protein
- HBSS:
-
Hank’s balanced salt solution
- Iba1:
-
Ionized calcium-binding adaptor molecule 1
- KL-OE:
-
Klotho-overexpressing
- IGF-1:
-
Insulin-like growth factor 1
- IHC:
-
Immunohistochemistry
- IL-1α:
-
Interleukin-1α
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- IL-12a:
-
Interleukin-12a
- iNOS:
-
Inducible nitric oxide synthase
- MAG:
-
Myelin-associated glycoprotein
- MBP:
-
Myelin basic protein
- MN:
-
Motor neurons
- MS:
-
Multiple sclerosis
- Prx-2:
-
Peroxiredoxin-2
- Prx-3:
-
Peroxiredoxin-3
- PLP1:
-
Proteolipid protein 1
- rmKL:
-
Recombinant mouse Klotho
- NeuN:
-
Neuronal nuclei
- NF-κB:
-
Nuclear factor-κB
- NOS:
-
Nitric oxide synthase
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NS:
-
Neurological score
- SMAD:
-
The activated type I receptors interact with and phosphorylate SMAD (an acronym for the fusion of Caenorhabditis elegans Sma genes and the Drosophila Mad) proteins to transduce signals
- SOD:
-
Superoxide dismutase
- TDP-43:
-
TAR DNA-binding protein 43
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor-α
- TNFAIP2:
-
TNF alpha-induced protein 2
- qRT-PCR:
-
Quantitative reverse transcription polymerase chain reaction
- VEGF:
-
Vascular endothelial growth factor
- Wnt:
-
Wnt is an acronym that stands for “Wingless/Integrated”
- WT:
-
Wild type
References
Abraham CR, Chen C, Cuny GD, Glicksman MA, Zeldich E (2012) Small-molecule Klotho enhancers as novel treatment of neurodegeneration. Future Med Chem 4:1671–1679
Abraham CR, Mullen PC, Tucker-Zhou T, Chen CD, Zeldich E (2016) Klotho is a neuroprotective and cognition-enhancing protein. Vitam Horm 101:215–238
Alexander GM, Erwin KL, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP, Heiman-Patterson TD (2004) Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res Mol Brain Res 130:7–15
Almer G, Vukosavic S, Romero N, Przedborski S (1999) Inducible nitric oxide synthase up-regulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem 72:2415–2425
Anamizu Y, Kawaguchi H, Seichi A, Yamaguchi S, Kawakami E, Kanda N, Matsubara S, Kuro-o M, Nabeshima Y, Nakamura K, Oyanagi K (2005) Klotho insufficiency causes decrease of ribosomal RNA gene transcription activity, cytoplasmic RNA and rough ER in the spinal anterior horn cells. Acta Neuropathol 109:457–466
Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, Robinson MD (2013) Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat Protoc 8:1765–1786
Baluchnejadmojarad T, Eftekhari SM, Jamali-Raeufy N, Haghani S, Zeinali H, Roghani M (2017) The anti-aging protein klotho alleviates injury of nigrostriatal dopaminergic pathway in 6-hydroxydopamine rat model of Parkinson’s disease: involvement of PKA/CaMKII/CREB signaling. Exp Gerontol 100:70–76
Bame M, Pentiak PA, Needleman R, Brusilow WS (2012) Effect of sex on lifespan, disease progression, and the response to methionine sulfoximine in the SOD1 G93A mouse model for ALS. Gend Med. 9:524–535
Beers DR, Zhao W, Liao B, Kano O, Wang J, Huang A, Appel SH, Henkel JS (2011) Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain Behav Immun 25:1025–1035
Behringer V, Stevens JMG, Deschner T, Sonnweber R, Hohmann G (2018) Aging and sex affect soluble alpha klotho levels in bonobos and chimpanzees. Front Zool 15:35
Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW (2006) Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 312:1389–1392
Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Goolsby H, Fontaine BA, Lemay N, McKenna-Yasek D, Frosch MP, Agar JN, Julien JP, Brady ST, Brown RH Jr (2010) Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci 13:1396–1403
Brobey RK, German D, Sonsalla PK, Gurnani P, Pastor J, Hsieh CC, Papaconstantinou J, Foster PP, Kuro-o M, Rosenblatt KP (2015) Klotho protects dopaminergic neuron oxidant-induced degeneration by modulating ASK1 and p38 MAPK signaling pathways. PLoS One 10:e0139914
Brown J, Pirrung M, McCue LA (2017) FQC dashboard: integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 33:3137–3139
Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z, Greco DJ, Wu PM, Doykan CE, Kiner O, Lawson RJ, Frosch MP, Pochet N, Fatimy RE, Krichevsky AM, Gygi SP, Lassmann H, Berry J, Cudkowicz ME, Weiner HL (2015) Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol 77:75–99
Cacabelos D, Ramirez-Nunez O, Granado-Serrano AB, Torres P, Ayala V, Moiseeva V, Povedano M, Ferrer I, Pamplona R, Portero-Otin M, Boada J (2016) Early and gender-specific differences in spinal cord mitochondrial function and oxidative stress markers in a mouse model of ALS. Acta Neuropathol Commun 4:3
Caldeira C, Oliveira AF, Cunha C, Vaz AR, Falcao AS, Fernandes A, Brites D (2014) Microglia change from a reactive to an age-like phenotype with the time in culture. Front Cell Neurosci 8:152
Chen Y, Guan Y, Zhang Z, Liu H, Wang S, Yu L, Wu X, Wang X (2012) Wnt signaling pathway is involved in the pathogenesis of amyotrophic lateral sclerosis in adult transgenic mice. Neurol Res 34:390–399
Chen CD, Sloane JA, Li H, Aytan N, Giannaris EL, Zeldich E, Hinman JD, Dedeoglu A, Rosene DL, Bansal R, Luebke JI, Kuro-o M, Abraham CR (2013) The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci 33:1927–1939
Chen CD, Zeldich E, Li Y, Yuste A, Abraham CR (2018) Activation of the anti-aging and cognition-enhancing gene klotho by CRISPR-dCas9 transcriptional effector complex. J Mol Neurosci 64:175–184
Cheng MF, Chen LJ, Niu HS, Yang TT, Lin KC, Cheng JT (2015) Signals mediating Klotho-induced neuroprotection in hippocampal neuronal cells. Acta Neurobiol Exp (Wars) 75:60–71
Cherry JD, Tripodis Y, Alvarez VE, Huber B, Kiernan PT, Daneshvar DH, Mez J, Montenigro PH, Solomon TM, Alosco ML, Stern RA, McKee AC, Stein TD (2016) Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun. 4:112
Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H (2008) Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci 268:40–47
Crosio C, Valle C, Casciati A, Iaccarino C, Carri MT (2011) Astroglial inhibition of NF-kappaB does not ameliorate disease onset and progression in a mouse model for amyotrophic lateral sclerosis (ALS). PLoS One 6:e17187
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 72:245–256
Devos D, Moreau C, Lassalle P, Perez T, De Seze J, Brunaud-Danel V, Destee A, Tonnel AB, Just N (2004) Low levels of the vascular endothelial growth factor in CSF from early ALS patients. Neurology. 62:2127–2129
Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M (2011) Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286:8655–8665
Dubal DB, Zhu L, Sanchez PE, Worden K, Broestl L, Johnson E, Ho K, Yu GQ, Kim D, Betourne A, Kuro OM, Masliah E, Abraham CR, Mucke L (2015) Life extension factor klotho prevents mortality and enhances cognition in hAPP transgenic mice. J Neurosci 35:2358–2371
Duce JA, Hollander W, Jaffe R, Abraham CR (2006) Activation of early components of complement targets myelin and oligodendrocytes in the aged rhesus monkey brain. Neurobiol Aging 27:633–644
Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR (2008) Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia. 56:106–117
German DC, Khobahy I, Pastor J, Kuro OM, Liu X (2012) Nuclear localization of Klotho in brain: an anti-aging protein. Neurobiol Aging 33(1483):e25–e30
Gill A, Kidd J, Vieira F, Thompson K, Perrin S (2009) No benefit from chronic lithium dosing in a sibling-matched, gender balanced, investigator-blinded trial using a standard mouse model of familial ALS. PLoS One 4:e6489
Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW (2006) Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci 26:8774–8786
Guo Y, Zhuang X, Huang Z, Zou J, Yang D, Hu X, Du Z, Wang L, Liao X (2018) Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-kappaB-mediated inflammation both in vitro and in vivo. Biochim Biophys Acta 1864:238–251
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX et al (1994) Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 264:1772–1775
Hall ED, Oostveen JA, Gurney ME (1998) Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 23:249–256
Halleskog C, Mulder J, Dahlstrom J, Mackie K, Hortobagyi T, Tanila H, Kumar Puli L, Farber K, Harkany T, Schulte G (2011) WNT signaling in activated microglia is proinflammatory. Glia. 59:119–131
Hatzipetros T, Kidd JD, Moreno AJ, Thompson K, Gill A, Vieira FG (2015) A quick phenotypic neurological scoring system for evaluating disease progression in the SOD1-G93A mouse model of ALS. J Vis Exp
Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH (2004) Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol 55:221–235
Hinman JD, Abraham CR (2007) What’s behind the decline? The role of white matter in brain aging. Neurochem Res 32:2023–2031
Hinman JD, Duce JA, Siman RA, Hollander W, Abraham CR (2004) Activation of calpain-1 in myelin and microglia in the white matter of the aged rhesus monkey. J Neurochem 89:430–441
Hinman JD, Peters A, Cabral H, Rosene DL, Hollander W, Rasband MN, Abraham CR (2006) Age-related molecular reorganization at the node of Ranvier. J Comp Neurol 495:351–362
Hui H, Zhai Y, Ao L, Cleveland JC Jr, Liu H, Fullerton DA, Meng X (2017) Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget. 8:15663–15676
Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Ogihara T (2006) Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun 339:827–832
Jana M, Pahan K (2013) Down-regulation of myelin gene expression in human oligodendrocytes by nitric oxide: implications for demyelination in multiple sclerosis. J Clin Cell Immunol 4
Jin M, Lv P, Chen G, Wang P, Zuo Z, Ren L, Bi J, Yang CW, Mei X, Han D (2017) Klotho ameliorates cyclosporine A-induced nephropathy via PDLIM2/NF-kB p65 signaling pathway. Biochem Biophys Res Commun 486:451–457
Kaneb HM, Sharp PS, Rahmani-Kondori N, Wells DJ (2011) Metformin treatment has no beneficial effect in a dose-response survival study in the SOD1(G93A) mouse model of ALS and is harmful in female mice. PLoS One 6:e24189
Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE (2013) Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16:571–579
King GD, Chen C, Huang MM, Zeldich E, Brazee PL, Schuman ER, Robin M, Cuny GD, Glicksman MA, Abraham CR (2012) Identification of novel small molecules that elevate Klotho expression. Biochem J 441:453–461
Krick S, Baumlin N, Aller SP, Aguiar C, Grabner A, Sailland J, Mendes E, Schmid A, Qi L, David NV, Geraghty P, King G, Birket SE, Rowe SM, Faul C, Salathe M (2017) Klotho inhibits interleukin-8 secretion from cystic fibrosis airway epithelia. Sci Rep 7:14388
Kuro-o M (2009) Klotho and aging. Biochim Biophys Acta 1790:1049–1058
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 390:45–51
Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M (2005) Suppression of aging in mice by the hormone Klotho. Science. 309:1829–1833
Lee DY, Jeon GS, Shim YM, Seong SY, Lee KW, Sung JJ (2015) Modulation of SOD1 subcellular localization by transfection with wild- or mutant-type SOD1 in primary neuron and astrocyte cultures from ALS mice. Exp Neurobiol. 24:226–234
Lee J, Hyeon SJ, Im H, Ryu H, Kim Y, Ryu H (2016) Astrocytes and microglia as non-cell autonomous players in the pathogenesis of ALS. Exp Neurobiol 25:233–240
Lewis KE, Rasmussen AL, Bennett W, King A, West AK, Chung RS, Chuah MI (2014) Microglia and motor neurons during disease progression in the SOD1G93A mouse model of amyotrophic lateral sclerosis: changes in arginase1 and inducible nitric oxide synthase. J Neuroinflammation 11:55
Li B, Xu W, Luo C, Gozal D, Liu R (2003) VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res Mol Brain Res 111:155–164
Li X, Guan Y, Chen Y, Zhang C, Shi C, Zhou F, Yu L, Juan J, Wang X (2013) Expression of Wnt5a and its receptor Fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. Int J Clin Exp Pathol 6:1245–1260
Liao B, Zhao W, Beers DR, Henkel JS, Appel SH (2012) Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol 237:147–152
Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 317:803–806
Masso A, Sanchez A, Bosch A, Gimenez-Llort L, Chillon M (2018) Secreted alphaKlotho isoform protects against age-dependent memory deficits. Mol Psychiatry 23(9):1937–1947
Matthews JN, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. BMJ. 300:230–235
McCombe PA, Henderson RD (2010) Effects of gender in amyotrophic lateral sclerosis. Gend Med 7:557–570
Molina M, Ortega G, Perez Gracia A, Saez JA (1989) Spontaneous bacterial empyema and hepatic cirrhosis. Enferm Infecc Microbiol Clin 7:516
Nagai T, Yamada K, Kim HC, Kim YS, Noda Y, Imura A, Nabeshima Y, Nabeshima T (2003) Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J 17:50–52
Needleman P, Manning PT (1999) Interactions between the inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) pathways: implications for therapeutic intervention in osteoarthritis. Osteoarthr Cartil 7:367–370
Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van Dorpe J, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P (2001) Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet 28:131–138
Paganoni S, Macklin EA, Lee A, Murphy A, Chang J, Zipf A, Cudkowicz M, Atassi N (2014) Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener 15:453–456
Parakh S, Atkin JD (2016) Protein folding alterations in amyotrophic lateral sclerosis. Brain Res 1648:633–649
Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM (2013) Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem 46:1079–1083
Peters OM, Ghasemi M, Brown RH Jr (2015) Emerging mechanisms of molecular pathology in ALS. J Clin Invest 125:1767–1779
Philips T, Bento-Abreu A, Nonneman A, Haeck W, Staats K, Geelen V, Hersmus N, Kusters B, Van Den Bosch L, Van Damme P, Richardson WD, Robberecht W (2013) Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 136:471–482
Pokrishevsky E, Hong RH, Mackenzie IR, Cashman NR (2017) Spinal cord homogenates from SOD1 familial amyotrophic lateral sclerosis induce SOD1 aggregation in living cells. PLoS One 12:e0184384
Razzaque MS (2012) The role of Klotho in energy metabolism. Nat Rev Endocrinol 8:579–587
Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Consortium I, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 72:257–268
Ross EK, Winter AN, Wilkins HM, Sumner WA, Duval N, Patterson D, Linseman DA (2014) A cystine-rich whey supplement (Immunocal((R))) delays disease onset and prevents spinal cord glutathione depletion in the hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. Antioxidants (Basel) 3:843–865
Samms RJ, Cheng CC, Kharitonenkov A, Gimeno RE, Adams AC (2016) Overexpression of beta-klotho in adipose tissue sensitizes male mice to endogenous FGF21 and provides protection from diet-induced obesity. Endocrinology. 157:1467–1480
Saura J, Tusell JM, Serratosa J (2003) High-yield isolation of murine microglia by mild trypsinization. Glia. 44:183–189
Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L (2011) Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc 59:1596–1601
Shiozaki M, Yoshimura K, Shibata M, Koike M, Matsuura N, Uchiyama Y, Gotow T (2008) Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience. 152:924–941
Shvil N, Banerjee V, Zoltsman G, Shani T, Kahn J, Abu-Hamad S, Papo N, Engel S, Bernhagen J, Israelson A (2018) MIF inhibits the formation and toxicity of misfolded SOD1 amyloid aggregates: implications for familial ALS. Cell Death Dis 9:107
Sloane JA, Hinman JD, Lubonia M, Hollander W, Abraham CR (2003) Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. J Neurochem 84:157–168
Solomonov Y, Hadad N, Levy R (2016) Reduction of cytosolic phospholipase A2alpha upregulation delays the onset of symptoms in SOD1G93A mouse model of amyotrophic lateral sclerosis. J Neuroinflammation 13:134
Sreedharan J (2010) Neuronal death in amyotrophic lateral sclerosis (ALS): what can we learn from genetics? CNS Neurol Disord Drug Targets 9:259–267
Tosolini AP, Sleigh JN (2017) Motor neuron gene therapy: lessons from spinal muscular atrophy for amyotrophic lateral sclerosis. Front Mol Neurosci 10:405
Wang YA (2006) Klotho, the long sought-after elixir and a novel tumor suppressor? Cancer Biol Ther 5:20–21
Weydt P, Hong SY, Kliot M, Moller T (2003) Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport. 14:1051–1054
Xin YJ, Yuan B, Yu B, Wang YQ, Wu JJ, Zhou WH, Qiu Z (2015) Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Sci Rep 5:7645
Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M (2005) Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280:38029–38034
Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 11:251–253
Yu L, Guan Y, Wu X, Chen Y, Liu Z, Du H, Wang X (2013) Wnt signaling is altered by spinal cord neuronal dysfunction in amyotrophic lateral sclerosis transgenic mice. Neurochem Res 38:1904–1913
Zeldich E, Koren R, Nemcovsky C, Weinreb M (2007) Enamel matrix derivative stimulates human gingival fibroblast proliferation via ERK. J Dent Res 86:41–46
Zeldich E, Chen CD, Colvin TA, Bove-Fenderson EA, Liang J, Tucker Zhou TB, Harris DA, Abraham CR (2014) The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem 289:24700–24715
Zeldich E, Chen CD, Avila R, Medicetty S, Abraham CR (2015) The anti-aging protein Klotho enhances remyelination following cuprizone-induced demyelination. J Mol Neurosci 57:185–196
Zhou HJ, Li H, Shi MQ, Mao XN, Liu DL, Chang YR, Gan YM, Kuang X, Du JR (2017) Protective effect of Klotho against ischemic brain injury is associated with inhibition of RIG-I/NF-kappaB signaling. Front Pharmacol 8:950
Zhou HJ, Zeng CY, Yang TT, Long FY, Kuang X, Du JR (2018) Lentivirus-mediated klotho up-regulation improves aging-related memory deficits and oxidative stress in senescence-accelerated mouse prone-8 mice. Life Sci 200:56–62
Zhu L, Stein LR, Kim D, Ho K, Yu GQ, Zhan L, Larsson TE, Mucke L (2018) Klotho controls the brain-immune system interface in the choroid plexus. Proc Natl Acad Sci U S A 115:E11388–E11396
Acknowledgments
The authors wish to thank Dr. Douglas Rosene for his help with statistical analysis.
Funding
This work was supported by NIH grants R01-AG052465 to NCL and R56-AG051638 to CRA.
Author information
Authors and Affiliations
Contributions
EZ, CDC, and CRA conceived the project. EZ, TH, and CRA designed the study. EZ, CDC, EB, BH, JSN, DZ, AGL, and AY performed the experiments in the laboratory of CRA and JDC and RMM in the laboratory of ACM. NCL and QM participated in the RNAseq analyses. All authors contributed to the analyses and/or interpreted the data. EZ wrote the paper with contributions from JSN, TH, and CRA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
All animal procedures were performed in accordance with a protocol approved by the Boston University Institutional Animal Care and Use Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeldich, E., Chen, CD., Boden, E. et al. Klotho Is Neuroprotective in the Superoxide Dismutase (SOD1G93A) Mouse Model of ALS. J Mol Neurosci 69, 264–285 (2019). https://doi.org/10.1007/s12031-019-01356-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01356-2